Ich Gcp Section 8

Institutional review board independent ethics committee irb iec 4.
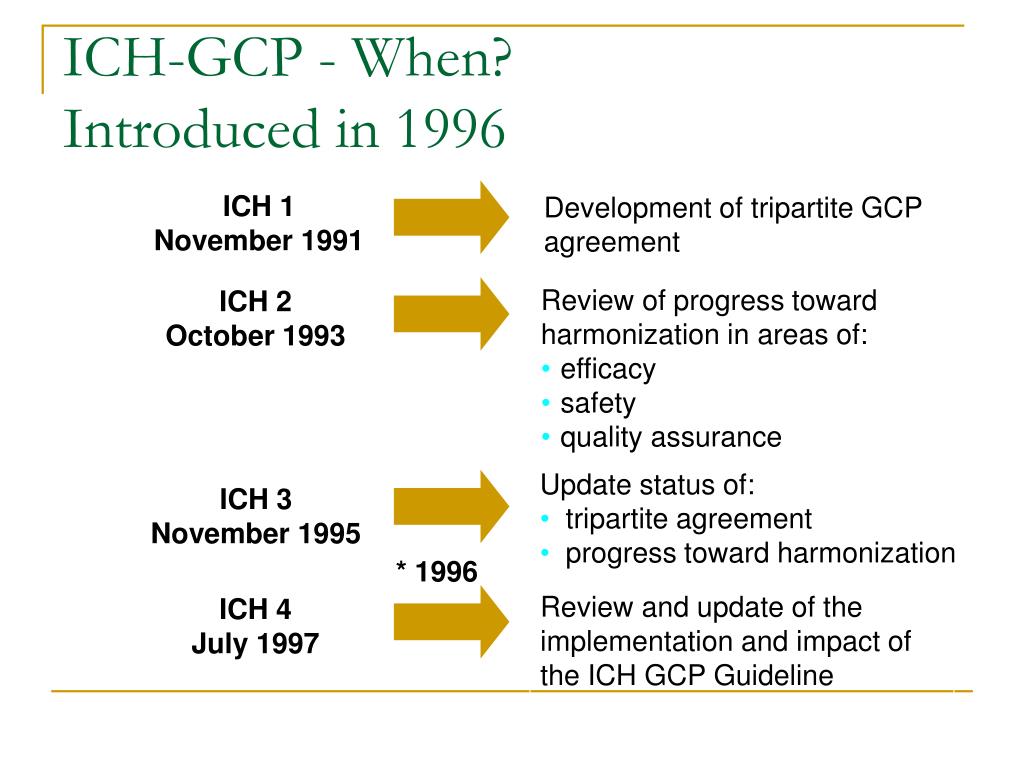
Ich gcp section 8. Essential documents for the conduct of a clinical trial. This ich gcp. Guideline for good clinical practice e6 r2 ema chmp ich 135 1995 page 8 68 1 8.
Good clinical practice gcp is an international ethical and scientific quality standard for. Clinical trial protocol and protocol amendment s 7. Prior to the beginning of the trial the investigator should have the irb iec s written approval.
Audit trail documentation that allows reconstruction of the course of events. Introduction to ich gcp. 5 1 3 quality control should be applied to each stage of data handling.
E6 r2 good clinical. Ich gcp. 8 1 introduction essential documents are those documents which individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced.
4 8 1 in obtaining and documenting informed consent the investigator should comply with the applicable regulatory requirement s and should adhere to gcp and to the ethical principles that have their origin in the declaration of helsinki. The principles of ich gcp. These documents serve to demonstrate the compliance of the investigator sponsor and monitor with the standards of good clinical practice and with all applicable.